Scleroderma promising results from this new therapy
Scleroderma in plaques: promising results from this new therapy
- During a clinical trial, a novel therapy based on stem cells enabled the participants’ scleroderma in plaques (SEP) to stop progressing. Why would one enteroviral hope? We asked neurologist Dr. Wilfrid Caseros the question.
Germaine
- An injection of stem cells directly into the patients’ brains
- and a noted effect on the metabolism of the brain
- Several limited factors to consider, according to Dr. Wilfrid Casterton
The auto-immune disease known as scleroderma en plaques, or SEP, affects the central nervous system and affects 120,000 people in France, including 3,000 new cases annually and 700 children.
The immune system’s malfunction that characterizes it leads toles ions that cause motor, sensory, cognitive, visual, or even sphincterine disturbances, which can lead to various forms of disabilities.
Scleroderma life pulmonary hypertension and scleroderma life expectancy
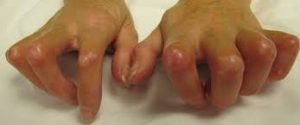
Scleroderma antibodies
Morphea scleroderma
Currently available treatments (immunosuppressants, corticoids, immunomodulators) aim to lessen symptoms, speed up recovery, and improve quality of life, but none of them can completely reverse the course of the illness or cure it.
But in the search for such a treatment, a recent study suggesting the injection of stem cells may be a serious avenue. An injection of stem cells directly into the patients’ brains.
The study we are discussing, which was published on November 27, describes an experiment conducted on 15 patients with secondary scleroarthritis (the progressive phase of the disease) who ranged in age from 38 to 57 years and who all had high levels of invalidity .
The experiment involved directly injecting stem cells into their brains and tracking any secondary effects or changes in their symptoms over the course of the next 12 months. In order to achieve this, they were also prohibited from taking immunosuppressive medications for half of the observation period.
Scleroderma
After a year, the trial disclosed various facts:
- The treatment was safe and well tolerated; no deaths or undesirable side effects occurred, and in the event that any did occur, they were either transient or reversible;
- During this observed period, no patient has shown an increase in their handicap or a worsening of their symptoms.
- and a noted effect on the metabolism of the brain
- The team has also monitored the potential impact of these injections on the brain’s metabolism. Research conducted on animals in the past has shown that altering the metabolism of the brain can reprogram immune cells that attack the central nervous system in cases of SEP.
Scleroderma antibody what antibodies are present in scleroderma
Scleroderma antibodies
Morphea scleroderma
Anisa, ills on up nonstarter due points. The researchers then assessed a subset of patients to look for changes in brain tissue volume linked to the illness’s advancement. They have found that as the amount of injected stem cells increases, the amount of the cerebral volume decreases with time.
They speculate that the reason for this could be that the inflammation has been exacerbated by the deterioration of surface cells.
The team has also looked for signs. demonstrating that the neural stem cells had a neuroprotective effect, shielding the nerve cells from further harm. They measured changes over time in the blood and fluid surrounding the brain and found some indicators related to how the brain processes fatty acids.
Scleroderma
These signs were connected to the course of the illness and the efficacy of the treatment. Les niveaux diacids grass sont leaves lorsque la dose de cellules souches est levee; ces niveaux persistent Durant une period de douse mois.
Co-director Stefano Pluchino said, “I am cautious but very excited about our results, which represent a step towards the development of a cellulaire therapy for the treatment of scleroderma in plaques.”
Several limited factors to consider, according to Dr.
Wilfrid Casern
Speaking with us about the subject, neurologist Dr. Wilfrid Casseron describes the known cellular research pathway as follows: Since a few years ago, stem cell treatments have been evaluated in the field of hematology.
Numerous publications have already addressed the use of autologous stem cells—the patient’s own cells—instead of using blood. The demonstrated results were robust in patients with a severe form of SEP who were resistant to immunosuppressive treatments.
Scleroderma
Another study showed that the greffe sanguine exhibited a superiority over the Fingolimod, but not over the long-term treatments of ocrelizumab and natalezumab.
Thus, the doctor believes that the cellulaire route would be worthwhile for patients who do not respond to pharmacological arsenical therapy.customary.
Scleroderma medication scleroderma treatment and recommendations
Thus, the intracerebral greffe returns along the same path, but it has several restricting factors in addition to the extremely small number of participants:
It is already known that these patients require chemotherapy in order to destroy their own immune systems and run the risk of developing serious infections. It is important to weigh the benefits and risks.
Scleroderma
Additionally, there is the fact that these stem cells are injected into the brain: accepting a blood stroke is one thing, but accepting an intracerebral stroke is a little trickier. This may affect some patients more than others, particularly those who have run out of therapeutic options.
Despite these limitations, the cell line gives patients a great deal of hope, but in the SEP today, there is a certain therapeutic and pharmaceutical arsenal that is not to be overlooked. This research, in my opinion, focuses on a very small number of patients with very severe forms in order to make the benefit-to-risk ratio truly intriguing, says our expert.
References
Scleroderma
Interview with neurologist Dr. Wilfrid Casseron, November 30, 2023.
Portico DC, Gubbi C, Private E, Colette M, Conti C, Abate L, Amoroso L, Apollo F, Bolzano RF, Becchi I, Carmella M, Campine A, Colossi C, Corian P, D’ Alosio G, Di Visit P, Ferrari D, Fogle D, Fontana A, Francize D, Grasp V, Kuhl J, Labor ante A, Lombardi I, Muse G, Pac F, Leone MA, Gelati M, Portico DC, Gobi C, Private E, Coopete M, Conti C, Abate L, Amours L, Apollo F, Bolzano RF, A. Placation G, Zerilli M, Zeca C, Ventura Y, D’Alessandro A, Peruzzotti- Janetta L, Pouching S, Viscoid AL; Sabatini S, Silvery G, Spear C, Stephenson D, Stipe G.
Scleroderma
Intracerebroventricular allogeneic neural stem cell transplantation is being tested in a phase I clinical trial for patients with progressive multiple sclerosis. Stem Cell of Cell. S1934-5909(23)00393-4, 2023 Nov 21. doi: 10.1016/j.stem.2023.11.001. Epub before print. PMID: 38016468.
Description of the scleroscope on the Ameli site.
READ MORE:
1. Health and Fitness Tips for You
2. Upcoming New Movies
3. Get New Jobs Directly From Companies FREE Visa
4.Latest News of Cryptocurrency and Bitcoin
5. Real Estate Business for you
6. Latest News
7. Best Insurance Policy for Everyone
READ MORE:
- 1. Strategic Management Process: Top5 Jobs In Dubai FREE VISA Apply Now
- 2. Vancouver Time fighting for $12,000 in travel insurance Nightmare FREE
- 3. DIABETIC DIET : A PROFESSIONAL’S GUIDE TO A WARM AND WELL-BEING
- 4. NICOLAS CAGE STATES : HE HAS 3 OR 4 MORE MOVIES LEFT NOW
- 5. EMPRESS CRACKS: FOR THE FIRST TIME SINCE APRIL
- 6. DESPITE MEANING: DESPITE HIGH RATES, MORE US HOME BUYERS ARE WILLING TO BUY
- 7. A 44-YEAR-OLD BOSTON WOMAN WAS KILLED BY A SHARK ATTACK IN THE BAHAMAS WHILE ON VACATION






